What is Optune Lua® approved to treat?
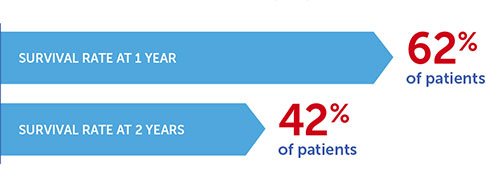
Optune Lua is a wearable, portable, FDA-approved device indicated for the treatment of adult patients, with unresectable, locally advanced or metastatic, malignant pleural mesothelioma (MPM) to be used together with standard chemotherapy (pemetrexed and platinum-based chemotherapy).
What is Optune® approved to treat?
Optune is a wearable, portable, FDA-approved device indicated to treat a type of brain cancer called glioblastoma multiforme (GBM) in adult patients 22 years of age or older.
Newly diagnosed GBM
If you have newly diagnosed GBM, Optune is used together with a chemotherapy called temozolomide (TMZ) if:
- Your cancer is confirmed by your healthcare professional AND
- You have had surgery to remove as much of the tumor as possible
Recurrent GBM
If your tumor has come back, Optune can be used alone as an alternative to standard medical therapy if:
- You have tried surgery and radiation and they did not work or are no longer working AND
- You have tried chemotherapy and your GBM has been confirmed by your healthcare professional
Who should not use Optune Lua for MPM or Optune for GBM?
Optune Lua for MPM and Optune for GBM are not for everyone. Talk to your doctor if you have:
- MPM and an implanted electronic medical device including a pacemaker, implantable automatic defibrillator, etc. Optune Lua has not been tested in people with implanted electronic devices, which may cause the devices not to work properly
- GBM and an implanted medical device (programmable shunt), skull defect (missing bone with no replacement) or bullet fragment. Optune has not been tested in people with implanted electronic devices, which may cause the devices not to work properly, and Optune has not been tested in people with skull defects or bullet fragments, which may cause Optune not to work properly
- A known sensitivity to conductive hydrogels (the gel on the arrays placed on the scalp or upper body like the ones used on EKGs). When Optune Lua/Optune comes into contact with the skin, it may cause more redness and itching or may rarely cause a life-threatening allergic reaction
Do not use Optune Lua for MPM or Optune for GBM if you are pregnant or are planning to become pregnant. It is not known if Optune Lua/Optune is safe or effective during pregnancy.
What should I know before using Optune Lua for MPM or Optune for GBM?
Optune Lua and Optune should only be used after receiving training from qualified personnel, such as your doctor, a nurse, or other medical staff who have completed a training course given by Novocure®, the maker of Optune Lua and Optune.
- Do not use any parts that did not come with Optune Lua or Optune Treatment Kit sent to you by Novocure or given to you by your doctor
- Do not get the device or transducer arrays wet
- Please be aware that Optune Lua and Optune have a cord that may cause tripping when connected to an electric socket
- If you have an underlying serious skin condition where the transducer arrays are placed, discuss with your doctor whether this may prevent or temporarily interfere with Optune Lua or Optune treatment
What are the possible side effects of Optune Lua for MPM and Optune for GBM?
The most common side effects of Optune Lua when used together with chemotherapy for MPM (pemetrexed and platinum-based chemotherapy) were low red blood cell count, constipation, nausea, tiredness, chest pain, fatigue, skin irritation from device use, itchy skin, and cough.
Other potential adverse effects associated with the use of Optune Lua include: treatment related skin irritation, allergic reaction to the plaster or to the gel, electrode overheating leading to pain and/or local skin burns, infections at sites of electrode contact with the skin, local warmth and tingling sensation beneath the electrodes, muscle twitching, medical device site reaction and skin breakdown/skin ulcer.
The most common side effects of Optune when used together with chemotherapy for GBM (temozolomide or TMZ) were low blood platelet count, nausea, constipation, vomiting, tiredness, seizure, and depression.
The most common side effects when using Optune alone for GBM were scalp irritation (redness and itchiness) and headache. Other side effects were malaise, muscle twitching, fall and skin ulcers.
Talk to your doctor if you have any of these side effects or questions.
Caution: Federal law restricts Optune Lua to sale by or on the order of a physician. Humanitarian Device. Authorized by Federal Law for use in the treatment of adult patients with unresectable, locally advanced or metastatic, malignant pleural mesothelioma concurrently with pemetrexed and platinum-based chemotherapy. The effectiveness of this device for this use has not been demonstrated.
Please click here to see the Optune Lua Instructions For Use (IFU) for complete information regarding the device's indications, contraindications, warnings, and precautions.
Please click here to see the Optune IFU for complete information regarding the device's indications, contraindications, warnings, and precautions.
On this site, patient and healthcare professional videos as well as all images labeled as Optune Lua users, caregivers, or healthcare professionals depict actual patients, caregivers, and healthcare professionals. All other depictions of patients and caregivers are actor portrayals. Patient images reflect the health status of the patients at the time each photo was taken.