STELLAR was a phase 2, prospective, pivotal, single-arm study1
STELLAR was a prospective, multicenter, single-arm, phase 2 study designed to study the safety and efficacy of Optune Lua and pemetrexed + cisplatin or carboplatin first line in patients with unresectable, locally advanced or metastatic, malignant pleural mesothelioma (MPM).
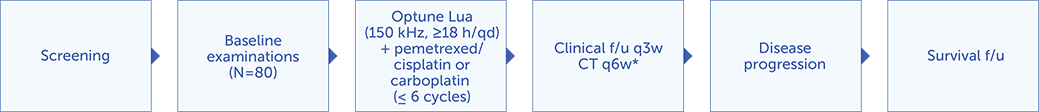
*CT of the chest and MRI and/or bone scan (if clinically indicated) were performed every 6 weeks until progression with a minimum follow-up of 12 months.
Phase 2 studies can be used for the basis of approval for making applications such as HDE. In the STELLAR study, all patients received the experimental treatment. This had been particularly important in the treatment of MPM, where the standard of care and prognosis had not changed for many years.1-4
Optune Lua was FDA approved under the Humanitarian Device Exemption (HDE) pathway and is classified as an Humanitarian Use Device (HUD)1,5
Study population1
Eighty (N=80) patients with unresectable and previously untreated MPM who were candidates for treatment with pemetrexed + cisplatin or carboplatin
Primary endpoint1
Overall survival (OS)
Secondary endpoints1
Progression-free survival (PFS), 1-year and 2-year survival rates, radiological response rate, and safety
Patient selection and baseline characteristics1
Major inclusion criteria
- TNM Stage IV, not candidate for curative treatment (surgery or radiotherapy)*
- ECOG Performance Status of 0-1
- ≥18 years old
- At least a 3-month life expectancy
- Measurable disease per mRECIST 1.1
Major exclusion criteria
- Previous chemotherapy or radiation
- Brain metastases (unless asymptomatic, pretreated, and not requiring steroids)
- Prior malignancy requiring anti-tumor treatment or concurrent malignancy
- Significant comorbidity impacting patient’s ability to receive systemic therapy
- Any implanted electronic device
- Significant comorbidity resulting in inadequate hematological, renal, or hepatic function, coagulopathy, or severe acute infection
*Patients with unresectable tumors were considered Stage IV at the time the protocol was written. Per a revision of the AJCC staging system in 2017, patients with unresectable tumors may be currently classified in either a Stage IIIB or Stage IV prognostic group.6
Patient baseline characteristics1,5
| Characteristics | Optune Lua and pemetrexed + cisplatin or carboplatin (N=80) |
|---|---|
| Median age, years (range) | 67 (27 - 78) |
| Sex, no. (%) Female Male |
13 (16%) 67 (84%) |
| Tumor stage, no. (%) Locally advanced Metastatic |
67 (84%) 13 (16%) |
| Tumor pathology, no. (%) Epithelioid Sarcomatoid/biphasic Unknown |
53 (66%) 21 (26%) 6 (8%) |
| ECOG performance status, no. (%) 0 1 |
45 (56%) 35 (44%) |
The 2019 STELLAR publication is available through Lancet Oncology
Ceresoli GL, Aerts JG, Dziadziuszko R, et al. Tumour Treating Fields in combination with pemetrexed and cisplatin or carboplatin as first-line treatment for unresectable malignant pleural mesothelioma (STELLAR): a multicentre, single-arm phase 2 trial. Lancet Oncol. 2019;20(12):1702-1709. doi:10.1016/S1470-2045(19)30532-7
ECOG, Eastern Cooperative Oncology Group; f/u, follow-up; mRECIST, modified Response Evaluation Criteria in Solid Tumors; qd, every day; q3w, every 3 weeks; q6w, every 6 weeks; TNM, tumor, node, metastasis.
References: 1. Optune Lua. Instructions for Use for Unresectable Malignant Pleural Mesothelioma. Novocure; 2021. 2. US FDA. Humanitarian Device Exemption (HDE) Program: Draft Guidance for Industry and Food and Drug Administration Staff. September 6, 2019. https://www.fda.gov/downloads/MedicalDevices/DeviceRegulationandGuidance/GuidanceDocuments/UCM389275.pdf. Accessed January 3, 2020. 3. Hazarika M, White RM, Johnson JR, Pazdur R. FDA drug approval summaries: pemetrexed (Alimta®). The Oncologist. 2004;9(5):482-488. 4. Vogelzang NJ, Rusthoven JJ, Symanowski J, et al. Phase III study of pemetrexed in combination with cisplatin versus cisplatin alone in patients with malignant pleural mesothelioma. J Clin Oncol. 2003;21(14):2636-2644. 5. Ceresoli GL, Aerts JG, Dziadziuszko R, et al. Tumour Treating Fields in combination with pemetrexed and cisplatin or carboplatin as first-line treatment for unresectable malignant pleural mesothelioma (STELLAR): a multicentre, single-arm phase 2 trial. Lancet Oncol. 2019;20(12):1702-1709. doi:10.1016/S1470-2045(19)30532-7 6. Amin MB, et al, eds. AJCC Cancer Staging Manual (8th edition). Springer International Publishing: American Joint Commission on Cancer; 2017.
