Optune Lua delivers Tumor Treating Fields (TTFields)1
- TTFields are low-intensity (1-3 V/cm), intermediate-frequency (200 kHz), alternating electric fields delivered to disrupt cancer cell division
- TTFields disrupt cell division through physical interaction with key molecules during multiple phases of mitosis1,2
TTFields target dividing cells, leading to apoptosis
Metaphase — uniform electric field
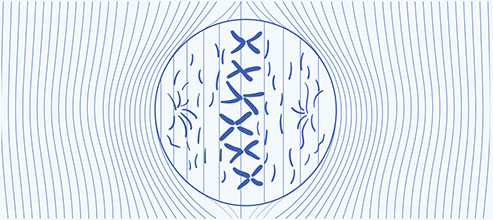
- Disrupt alignment of highly polarized tubulin subunits2
- Disrupt microtubule spindle formation during mitosis and may ultimately lead to apoptosis2,3
Telophase — nonuniform electric field
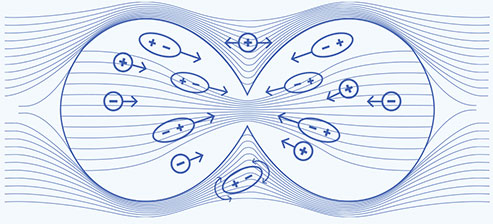
- A change in cell shape during telophase causes a nonuniform electric field2,4
- Polar cell components move toward cleavage furrow2
- Cell cannot divide properly, which may ultimately lead to apoptosis2,3
View TTFields mechanism of action video
This video showcases the detailed process of how TTFields disrupt cancer cell division.
Optune Lua has the same mechanism of action as Optune®1,5,6,*
Optune, which has been approved for recurrent glioblastoma multiforme (GBM) since 2011 and newly diagnosed GBM together with temozolomide (TMZ) since 2015, offers real-world experience and clinical evidence delivering TTFields in patients with both recurrent and newly diagnosed GBM.5,7
More than 18,000 patients have been treated with TTFields since the FDA approval of Optune in 20115,8
Newly diagnosed GBM*:
The addition of Optune to maintenance temozolomide (TMZ) significantly improved PFS and OS with QoL maintained over time, measured up to one year.†,7,8
Recurrent GBM‡:
Patients treated with Optune as monotherapy experienced similar efficacy, improved cognitive and emotional functioning, and fewer systemic AEs compared with physician’s choice of chemotherapy.6,9
- Optune was approved under the Premarket Authorization (PMA) pathway based on data from a phase 3 randomized controlled trial10
Optune Lua is FDA approved as first-line therapy together with pemetrexed and platinum-based chemotherapy for patients with MPM1,11
*EF-14 was a prospective, randomized, open-label, phase 3 clinical trial that was designed to evaluate the efficacy and safety of TTFields + TMZ vs maintenance TMZ in patients newly diagnosed with supratentorial glioblastoma who completed radiation therapy and adjuvant TMZ. Patients (N=695) were randomized in a 2 to 1 ratio to receive either TTFields + TMZ or TMZ alone. Treatment began 4 to 7 weeks after the end of chemotherapy and radiation therapy. The specific objectives of the study included: PFS (primary endpoint), OS (powered secondary endpoint), 1- and 2-year survival rate, overall response rate, QoL, and safety.11
†Patient-reported QoL data collected per EORTC QLQ-C30 at baseline and Months 3, 6, 9, and 12. The 30-question survey covered 5 daily-functioning domains (physical, role, social, emotional, and cognitive).7,8
‡EF-11 was a prospective, randomized, open-label, phase 3 clinical trial that was designed to evaluate the efficacy and safety of TTFields as a monotherapy vs physician’s best choice for chemotherapy (including bevacizumab) in patients with supratentorial recurrent GBM. The best available therapy was prescribed according to local practice and depending on prior treatment exposure. Adult patients (N=237) were randomized in a 1:1 manner to either TTFields or chemotherapy. The primary endpoint was OS. Secondary objectives included: PFS, 1-year survival rate, radiological response rate, QoL, and safety.11
AEs, adverse events; EORTC QLQ-C30, European Organization for Research and Treatment of Cancer core quality of life questionnaire; MPM, malignant pleural mesothelioma; OS, overall survival; PFS, progression-free survival; QoL, quality of life.
References: 1. Optune Lua. Instructions for Use for Unresectable Malignant Pleural Mesothelioma. Novocure; 2021. 2. Kirson ED, Gurvich Z, Schneiderman R, et al. Disruption of cancer cell replication by alternating electric fields. Cancer Res. 2004;64(9):3288-3295. 3. Giladi M, Schneiderman RS, Voloshin T, et al. Mitotic spindle disruption by alternating electric fields leads to improper chromosome segregation and mitotic catastrophe in cancer cells. Scientific Reports. 2015;5:18046. 4. Gutin PH, Wong ET. Noninvasive application of alternating electric fields in glioblastoma: a fourth cancer treatment modality. Am Soc Clin Oncol Educ Book. 2012:126-131. 5. Summary of Safety and Effectiveness Data (SSED). US FDA Website. https://www.accessdata.fda.gov/cdrh_docs/pdf10/p100034s013b.pdf. Accessed January 3, 2020. 6. Optune. Instructions for Use for Glioblastoma Multiforme. Novocure; 2019. 7. Stupp R, Taillibert S, Kanner A, et al. Effect of Tumor-Treating Fields plus maintenance temozolomide vs maintenance temozolomide alone on survival in patients with glioblastoma: a randomized clinical trial. JAMA. 2017;318(23):2306-2316. 8. Taphoorn MJB, Dirven L, Kanner AA, et al. Influence of treatment with tumor-treating fields on health-related quality of life of patients with newly diagnosed glioblastoma: a secondary analysis of a randomized clinical trial. JAMA Oncol. 2018;4(4):495-504. doi:10.1001/jamaoncol.2017.5082. 9. Stupp R, Wong ET, Kanner AA, et al. NovoTTF-100A versus physician’s choice chemotherapy in recurrent glioblastoma: a randomised phase III trial of a novel treatment modality. Eur J Cancer. 2012;48(14):2192-2202. 10. US Food and Drug Administration. Premarket Approval (PMA) Database. Silver Spring, MD: US Department of Health and Human Services; 2015. 11. FDA Approves the NovoTTF-100L™ System in Combination with Chemotherapy for the Treatment of Malignant Pleural Mesothelioma [press release]. St. Helier, Jersey: Business Wire; May 23, 2019.
