STELLAR Clinical Results: Patients who used Optune Lua first line together with pemetrexed + cisplatin or carboplatin achieved 18.2 months median OS1,2
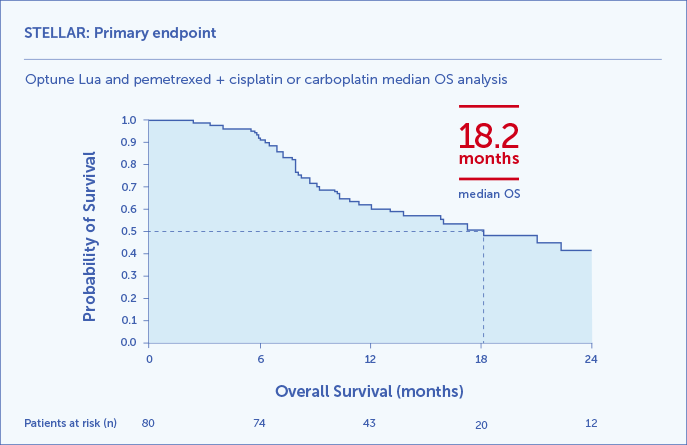
(95% CI, 12.1-25.8) The Kaplan Meier overall survival curve for the 80 study patients.
The STELLAR study was a prospective, single-arm, phase 2 trial to study the safety and efficacy of Optune Lua first line in patients with unresectable, locally advanced or metastatic, malignant pleural mesothelioma (N=80). Patients were ≥18 years of age, had an ECOG performance status of 0-1, and at least one measurable or evaluable lesion according to mRECIST for MPM. Patients received continuous TTFields to the thorax at a frequency of 150 kHz for at least 18 hours/day and concomitant chemotherapy every 21 days for up to 6 cycles.
Optune Lua and pemetrexed + cisplatin or carboplatin median OS results shown across histologies1,3
- 21.2 months median OS in 66% of patients with epithelioid histology (2/3 of participants)
- 12.1 months in 34% of patients with less responsive, harder to treat nonepithelioid histology (1/3 of participants)*
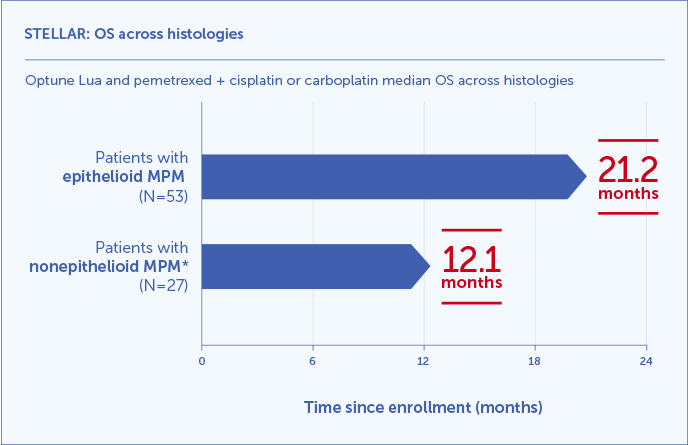
*Nonepithelioid histology includes sarcomatoid or biphasic and unknown tumor pathology.
STELLAR Clinical Results: Patients using Optune Lua achieved 7.6 months median progression-free survival (PFS) when used together with pemetrexed + cisplatin or carboplatin as first-line treatment (N=80)1,2
- 95% CI, 6.7-8.6, across all patients treated
Survival rates1,2
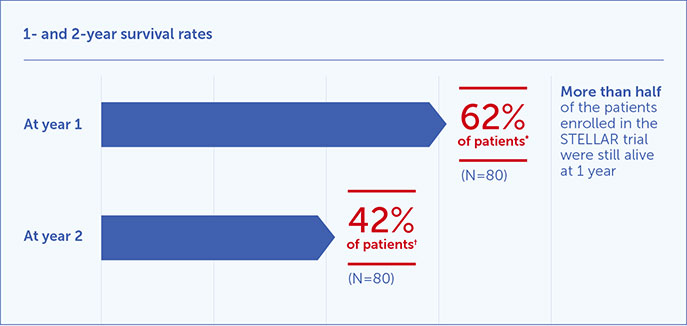
*95% CI: 50.3 - 72.0.
†95% CI: 28.0 - 55.2.
Radiological response rate1,2
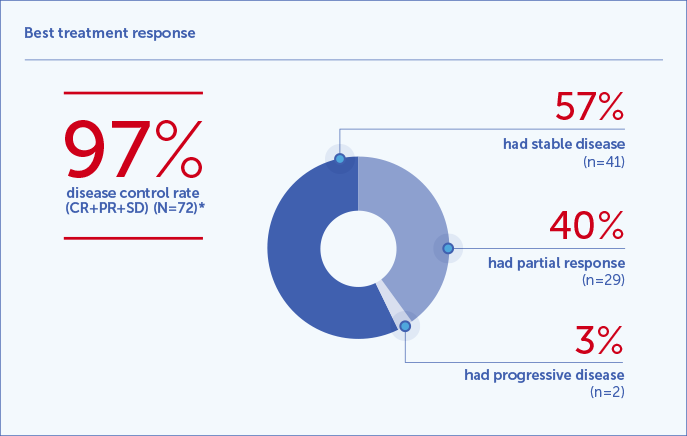
*In patients with at least one follow-up CT scan performed and were evaluable for response according to mRECIST criteria.
Optune Lua is FDA approved as first-line therapy for patients with MPM1,4
AE, adverse event; CR, complete response; CT, computed tomography; ECOG, Eastern Cooperative Oncology Group; mRECIST, modified Response Evaluation Criteria in Solid Tumors; MPM, malignant pleural mesothelioma; PR, partial response; SD, stable disease; TTFields, Tumor Treating Fields.
References: 1. Optune Lua. Instructions for Use for Unresectable Malignant Pleural Mesothelioma. Novocure; 2021. 2. Ceresoli GL, Aerts JG, Dziadziuszko R, et al. Tumour Treating Fields in combination with pemetrexed and cisplatin or carboplatin as first-line treatment for unresectable malignant pleural mesothelioma (STELLAR): a multicentre, single-arm phase 2 trial. Lancet Oncol. 2019;20(12):1702-1709. doi:10.1016/S1470-2045(19)30532-7 3. Mansfield AS, Symanowski JT, Peikert T. Systematic review of response rates of sarcomatoid malignant pleural mesotheliomas in clinical trials. Lung Cancer. 2014;86(2):133-136. 4. FDA Approves the NovoTTF-100L™ System in Combination with Chemotherapy for the Treatment of Malignant Pleural Mesothelioma [press release]. St. Helier, Jersey: Business Wire; May 23, 2019.
